Antibiotic and Anticoagulation: Watching Warfarin Levels
Retail health care providers are in an optimal position to monitor for drug interactions between warfarin and antibiotics because 80% to 90% of all antibiotics are prescribed in an outpatient setting.
Warfarin (Coumadin) is the most commonly prescribed anticoagulant to prevent thromboembolisms in patients with atrial fibrillation, venous thromboembolism, and prosthetic heart valves. Providers must closely monitor the degree of anticoagulation in patients on warfarin using the international normalized ratio (INR). Many patients have an INR target range between 2 and 3 or between 2.5 and 3.5, depending on the indication for anticoagulant therapy, ensuring appropriate anticoagulation and avoiding thrombosis and bleeds.1For patients to achieve an INR in their therapeutic range, they must maintain consistent vitamin K intake and adhere to their medication.2Providers must titrate warfarin doses appropriately and regularly review patients’ medications for possible interactions. A patient’s INR can increase or decrease with newly prescribed or discontinued medications. Retail health care providers are in an optimal position to monitor for drug interactions between warfarin and antibiotics because 80% to 90% of all antibiotics are prescribed in an outpatient setting.3
WHEN TO WORRY
Prescribers must consider how adding and removing medications will affect a patient’s INR. Medications that interact with warfarin often have a unidirectional effect on INR. If a patient’s INR is already stabilized on warfarin and an INR-increasing antibiotic but the antibiotic is discontinued, the patient’s INR could drop below the therapeutic range. Similarly, if a patient is controlled on warfarin but a provider begins an INR-increasing antibiotic, the patient’s INR could rise above the therapeutic range.1
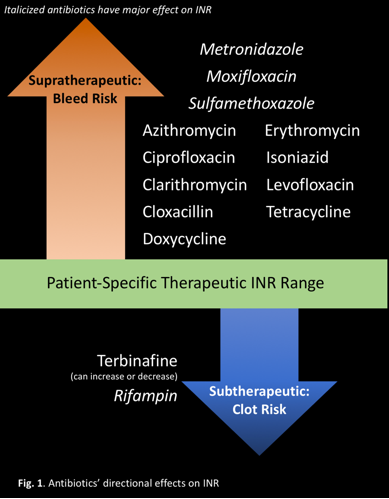
MANY INTERACTIONS, SEVERAL CAUSES
Warfarin is metabolized by the cytochrome (CYP) P450 enzyme system, specifically, CYPs 1A2, 3A4, and 2C9.2Medications that inhibit these CYP enzymes reduce warfarin's metabolism, increasing its effect, and increase INR. Medications that induce these CYP enzymes increase warfarin's metabolism, decreasing its therapeutic effect, and decrease INR. Medications can also directly affect the clotting cascade, induce warfarin's metabolism, or affect its protein binding. Warfarin is highly protein bound.2If other medications displace warfarin on protein-binding sites, the free concentration will increase, creating a bleeding risk.
Antibiotics in the same class have similar effects on INR:
- Cephalosporins may increase INR by inhibiting production of vitamin K-dependent clotting factors.6,13
- Fluoroquinolones may increase INR by inhibiting warfarin metabolism, displacing warfarin from protein-binding sites, or disturbing intestinal flora that synthesizes vitamin K.7,13
- Isoniazid may increase INR by inhibiting warfarin's metabolism.9,13
- Macrolides and metronidazole may increase INR by inhibiting warfarin's metabolism.4,8,13
- Penicillin may increase bleeding risk by inhibiting platelet function.12,13
- Rifampin may decrease INR by inducing warfarin's metabolism.10,13
- Sulfonamides may increase INR by inhibiting warfarin metabolism, displacing warfarin from protein-binding sites, or disturbing intestinal flora that synthesizes vitamin K.5,13
- Tetracyclines increase INR by an unknown mechanism, potentially inhibiting warfarin metabolism and plasma prothrombin activity.11,13
TAKE ACTION
Providers can gauge the clinical relevance of a drug interaction based on its anticipated onset and offset and dose-adjust accordingly. Specific clinically significant warfarin-antibiotic interactions are included in the table14with recommendations for whether and how a provider should change the warfarin dose.
TABLE.
DRUG INTERACTIONS BETWEEN WARFARIN AND COMMONLY PRESCRIBED ANTIBIOTICS
14
Drug
Direction and Severity of Effect on INR
Mechanism
Anticipated Onset
Anticipated Offset
AMS
†
Suggested Management
Metronidazole
Major increase in INR
Inhibits warfarin metabolism via CYP2C9
3 to 5 days
~2 days
Consider empiric 25% to 40% warfarin dose reduction.
Moxifloxacin
May inhibit
warfarin metabolism via CYP1A2+
2 to 5 days
2-3 days
Consider empiric 0% to 25% warfarin dose reduction.
Sulfamethoxazole
Inhibits warfarin metabolism, displaces protein binding
2 to 5 days
2-14 days
Consider empiric 25% to 40% warfarin dose reduction.
Azithromycin
Moderate increase in INR
Possibly decreases warfarin metabolism*
3 to 7 days
Not reported
Inconsistent effect; no dose change unless other factors affect INR.
Ciprofloxacin
May be due to CYP1A2 inhibition+
2 to 5 days
2-4 days
Consider empiric 10% to 15% warfarin dose reduction.
Clarithromycin
Inhibits warfarin metabolism via CYP3A4
3 to 7 days
Not reported
Consider empiric 15% to 25% warfarin dose reduction.
Cloxacillin
Unknown
Delayed
Not reported
Inconsistent effect; no dose change unless other factors affect INR.
Doxycycline
May inhibit
warfarin metabolism via CYP3A4
2 to 5 days
Not reported
Inconsistent effect; no dose change unless other factors affect INR.
Erythromycin
Inhibits warfarin metabolism via CYP3A4
3 to 5 days
3-5 days
Consider empiric 10% to 15% warfarin dose reduction.
Isoniazid
Inhibits warfarin metabolism via CYP2C9
3 to 5 days
Delayed
Consider empiric 10% to 15% warfarin dose reduction; monitor INR weekly.
Levofloxacin
May inhibit
warfarin metabolism via CYP1A2+
3 to 5 days
5-10 days
Consider empiric 0% to 15% warfarin dose reduction.
Tetracycline
Reduces plasma pro- thrombin activity
2 to 5 days
Not reported
Monitor INR closely.
Rifampin
Moderate to severe decrease in INR
Induces hepatic warfa- rin metabolism
1 to 3 weeks
1-5 weeks
Consider empiric 25% to 50% warfarin dose increase.**
Terbinafine
Can increase or decrease INR
Unknown
Unknown
Not reported
Monitor INR closely.

Retail health care providers are accessible and reliable and should monitor warfarin-treated patients carefully. Initiating and discontinuing antibiotic therapy, even for a short duration, may increase or decrease a patient's INR. The longer that INR is outside the therapeutic range the higher the risk is for bleeds, clots, and even further complications.
Emily Seamans is a PharmD candidate at the University of Connecticut School of Pharmacy in Storrs, Connecticut.
References
1. Wigle P, Hein B, Bloomfield HE, Tubb M, Doherty M. Updated guidelines on outpatient anticoagulation. Am Fam Physician.2013;87(8):556-66.2.
2. Coumadin [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Co; 2011.accessdata.fda.gov/drugsatfda_docs/label/2011/009218s107lbl.pdf. Accessed August 24, 2017.
3. CDC: Measuring outpatient antibiotic prescribing.cdc.gov/getsmart/community/programs-measurement/measuring-antibiotic-prescribing.html. Updated March 22, 2017. Accessed August 22, 2017.
4. Azithromycin [prescribing information]. New York, NY: Pfizer Labs; 2017.labeling.pfizer.com/ShowLabeling.aspx?id=511. Accessed August 24, 2017.
5. Bactrim [prescribing information]. Philadelphia, PA: Mutual Pharmaceutical Company Inc; 2013.accessdata.fda.gov/drugsatfda_docs/label/2003/17377slr057_Bactrim_lbl.pdf. Accessed August 24, 2017.
6. Rocephin [prescribing information]. Nutley, NJ: Roche Pharmaceuticals; 1998.accessdata.fda.gov/drugsatfda_docs/label/2009/0550585s063lbl.pdf. Accessed August 24, 2017.
7. Cipro [prescribing information]. West Haven, CT: Bayer Corp; 2000.dartmouth.edu/~genchem/0102/spring/6winn/pdfs/ciprotab.pdf. Accessed August 24, 2017.
8. Flagyl [prescribing information]. Chicago, IL: Pharmacia Corp; 2003.accessdata.fda.gov/drugsatfda_docs/label/2004/12623slr059_flagyl_lbl.pdf. Accessed August 24, 2017.
9. Rifater [prescribing information]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2010.accessdata.fda.gov/drugsatfda_docs/label/2010/050705s007lbl.pdf. Accessed August 24, 2017.
10. Rifadin [prescribing information]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2016.products.sanofi.us/rifadin/rifadin.pdf. Accessed August 24, 2017.
11. Tetracycline [prescribing information]. Eatontown, NJ: Heritage Pharmaceuticals Inc; 2015.accessdata.fda.gov/drugsatfda_docs/label/2015/050278s037lbl.pdf. Accessed August 24, 2017.
12. Unasyn [prescribing information]. New York, NY: Pfizer Inc; 2007.accessdata.fda.gov/drugsatfda_docs/label/2008/050608s029lbl.pdf. Accessed August 24, 2017.
13. PL Detail-Document. Antimicrobial drug interactions and warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012. file:///C:/Users/cmollison/Downloads/280823%20(1).pdf. Accessed August 24, 2017.
14. Bungard TJ, Yakiwchuk E, Foisy M, Brocklebank C. Drug interactions involving warfarin: practice tool and practical management tips.Can Pharm J.2011;144:21-25.doi.org/10.3821/1913-701X-144.1.21.

