3 Hep C Treatment Guideline Updates Retail Clinicians Should Know
The World Health Organization created its first hepatitis C treatment guidelines back in 2014, but it just released some updates that promote the use of newer and more effective medicines.
The World Health Organization (WHO) created its first hepatitis C virus (HCV) treatment guidelines back in 2014, but it just released some updates that promote the use of newer and more effective medicines.
The 2016 WHO guidelines for HCV treatment also highlight some of the best practices that continue to be recommended to this day. For example, WHO continues to advise health care providers to offer HCV screening to members of populations where HCV is prevalent and to those with a history of HCV risk exposure or behavior.
In addition, patients should be screened and counseled on reducing their alcohol consumption if they report high or moderate alcohol intake.
Around 130 million to 150 million individuals are infected with HCV worldwide each year, and around 700,000 individuals die from related complications.
Here’s what retail clinicians should know about WHO’s updated HCV treatment guidelines:
1. Direct-acting antivirals (DAAs) have been deemed crucial for curing HCV.
DAAs, which are more effective and easier to use than other established treatments (eg, pegylated interferon and ribavirin), have come to market since the 2014 HCV guidelines were published.
The basics of DAAs are that they’re easy to take (1 pill per day in some cases) and patients typically only have to take them for 8 to 12 weeks. DAAs are also associated with few adverse effects, and more than 90% of patients treated with them are cured of HCV.
“This is a vast improvement from older treatments, which cured less than half of the people treated, required weekly interferon injections for up to 12 months, and often resulted in severe, sometimes fatal, side effects,” a WHO press release noted.
However, WHO did note that patients with HCV genotype 3 infection with cirrhosis, as well as those with genotypes 5 and 6 infection with and without cirrhosis, can take an interferon-based regimen of sofosbuvir/pegylated interferon and ribavirin as an alternative treatment.
The new guidelines also remove the recommendation for boceprevir- or telaprevir-containing regimens for those with HCV.
In addition, WHO added DAAs to its Model List of Essential Medicines.
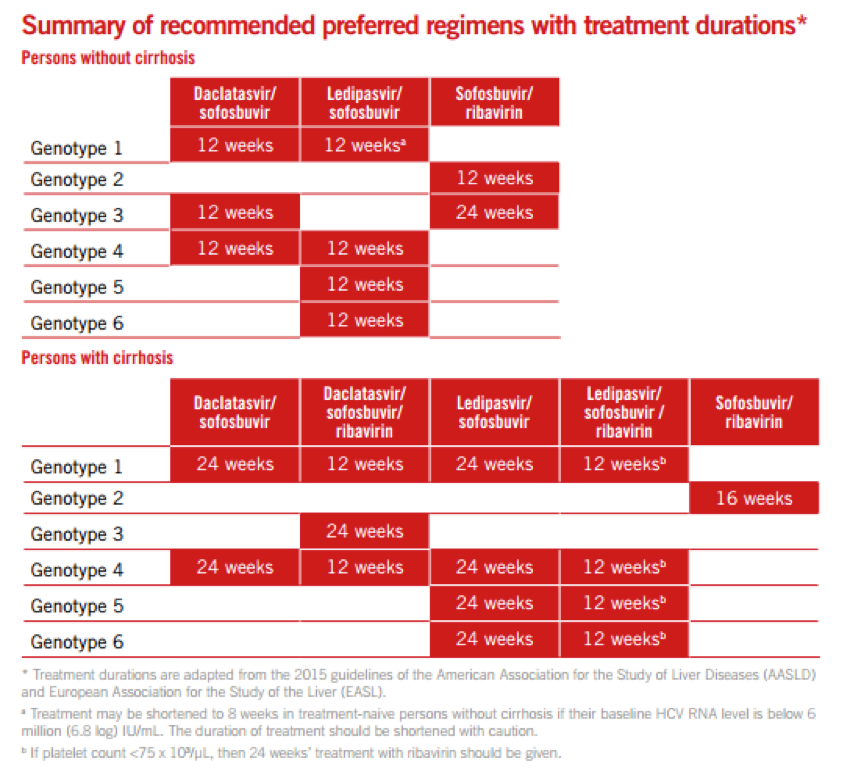
2. Preferred HCV treatment regimens have been simplified.
The new guidelines simplify which type of medication regimen a patient should take based on his or her clinical history and HCV genotype.
“The selection of preferred and alternative regimens remains somewhat complex; however, the recommended regimens in these guidelines are a step in the direction of recommending a single regimen for all genotypes and for all patients, regardless of the degree of cirrhosis and previous treatment experience,” the report stated.
3. HCV treatment accessibility in poorer areas has been emphasized.
WHO highlighted that patients who struggle financially in low- and middle-income areas aren’t able to access HCV treatment, but these are the same places where infection is most prominent.
Price and availability of DAAs also vary by country. In some high-income countries, treatment can cost $100,000, whereas generic versions of DAAs are available for less than $500 in places like India.
“WHO recognizes that implementation of the recommendations may not be immediate because the treatments can be expensive, and the medicines are not yet approved in many countries,” the press release stated.
