Rx Update: GlaxoSmithKline's Breo Ellipta Approved for Asthma

On April 30, 2015, GlaxoSmithKline announced FDA approval of Breo Ellipta (fluticasone furoate and vilanterol inhalation powder) as a once-daily treatment for asthma in patients aged 18 years and older.1Previously, since its initial approval in 2013, Breo Ellipta had only been indicated for use as a long-term once-daily maintenance treatment of airflow obstruction and reducing exacerbations in patients with chronic obstructive pulmonary disease (COPD).2
Breo Ellipta, like all products containing long-acting beta2-adrenergic agonists (LABAs), carries a black-box warning for asthma-related death. Current data are inadequate to determine whether use of inhaled corticosteroids mitigates the risk of asthma-related death associated with LABAs. Some data indicate that pediatric and adolescent patients using LABAs may be at increased risk for asthma-related hospitalization.2
Contraindications for use of Breo Ellipta include primary treatment of status asthmaticus or acute episodes of COPD or asthma that requires intensive measures for treatment. In addition, use of Breo Ellipta is contraindicated in patients with severe hypersensitivity to milk proteins or to any of the ingredients in the formulation.2
Mechanism of Action
Vilanterol, the LABA in Breo Ellipta, has a similar functional selectivity for beta2 receptors as salmeterol, based on in vitro studies. Vilanterol triggers relaxation of bronchial smooth muscle and inhibits inflammatory mediator release, especially from mast cells.2
Dosage and Administration
Breo Ellipta comes in 2 strengths: 100 mcg of fluticasone furoate and 25 mcg of vilanterol per inhalation or 200 mcg of fluticasone furoate and 25 mcg of vilanterol per inhalation. The recommended dose for treatment of both asthma and COPD is 1 inhalation daily.2
In patients with COPD, the only approved dosage of Breo Ellipta is 100 mcg/25 mcg once daily, whereas in patients with asthma, either Breo Ellipta strength of 100 mcg/25 mcg or 200 mcg/25 mcg strength may be used. In asthma, the appropriate starting dose should be chosen based on the severity of the patient’s asthma symptoms. No dosage adjustment is necessary in patients with renal impairment or hepatic impairment.2
Patients should be advised to rinse their mouth with water after inhalation, without swallowing the water, to reduce the risk of developing a fungal (Candida albicans) infection of the mouth or pharynx.2
Clinical Studies in Asthma
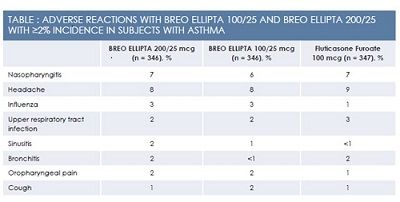
Breo Ellipta has been evaluated in a total of 9969 patients with asthma across 4 confirmatory clinical trials of 12 to 76 weeks’ duration. A comparative trial of Breo Ellipta versus fluticasone propionate/salmeterol 250 mcg/50 mcg lasted 24 weeks. Over 24 to 76 weeks, patients receiving Breo Ellipta at the 100‑mcg/25‑mcg daily dose experienced a significant decrease in the risk of asthma exacerbations and the time to first exacerbation compared with an active control group receiving fluticasone furoate 100 mcg daily. Improvements in the area under the FEV1‑versus‑time curve over 0 to 24 hours ranged from 77 mL to 193 mL, depending on the trial. Although children as young as age 12 years were enrolled in some of these trials, Breo Ellipta was approved for use in adults 18 years and older with asthma. Adverse events are summarized in the Table.2
Warnings and Precautions
Pneumonia and Infections
Patients using Breo Ellipta may be at increased risk of developing pneumonia, particularly patients with COPD. Patients should be monitored for signs of infection. Serious and even fatal outcomes have occurred in patients who have developed chickenpox or measles while using Breo Ellipta.2
Adrenal Suppression
Because Breo Ellipta contains a corticosteroid, there is a risk of impaired adrenal function, particularly when transitioning from a systemic corticosteroid to an inhaled corticosteroid. As a result, systemic corticosteroids should be tapered off slowly when patients transition from long-term systemic corticosteroid treatment to Breo Ellipta.2
Monitoring
Patients who experience paradoxical bronchospasm after using Breo Ellipta should use alternative therapy. In addition, patients with cardiovascular disorders, glaucoma, and at risk for cataracts should be monitored periodically while using Breo Ellipta. Patients with convulsive disorders, thyrotoxicosis, diabetes, and ketoacidosis should use Breo Ellipta with caution. Monitoring for hypokalemia and hyperglycemia is recommended.2
Drug-Drug Interactions
Strong inhibitors of cytochrome P450 3A4 enzymes may increase the effective levels of the medications in Breo Ellipta, potentially leading to systemic corticosteroid and cardiovascular effects. Patients taking monoamine oxidase inhibitors and tricyclic antidepressants should use Breo Ellipta with caution, as these medications may increase the patient’s sensitivity to vilanterol. In addition, beta-blockers may inhibit the bronchodilatory effects of vilanterol. Patients using diuretics should be monitored for hypokalemia when using beta-agonists, including vilanterol.2
Reproductive Hazards
Although there are no adequate and well-controlled trials with Breo Ellipta in pregnant women, based on animal reproduction studies, Breo Ellipta is a Pregnancy Category C medication. Breo Ellipta should only be used in pregnant women if the potential benefit justifies the potential risk to the fetus.2
Common Adverse Events
In patients using Breo Ellipta for asthma, common adverse events (defined as adverse events occurring with an incidence of 2% or higher) included nasopharyngitis, oral candidiasis, headache, influenza, upper respiratory tract infection, bronchitis, sinusitis, oropharyngeal pain, dysphonia, and cough. For a complete discussion of potential adverse events and drug interactions with Breo Ellipta, please consult the product package insert.2
References
1. Breo Ellipta [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2015.
2. FDA approves BREO ELLIPTA for the treatment of adults with asthma in the US [press release]. London, England, and San Francisco, CA: PR Newswire; April 30, 2015. www.prnewswire.com/news-releases/fda-approves-breo-ellipta-for-the-treatment-of-adults-with-asthma-in-the-us-300075582.html. Accessed May 2015.

