Product Updates: Rx Products
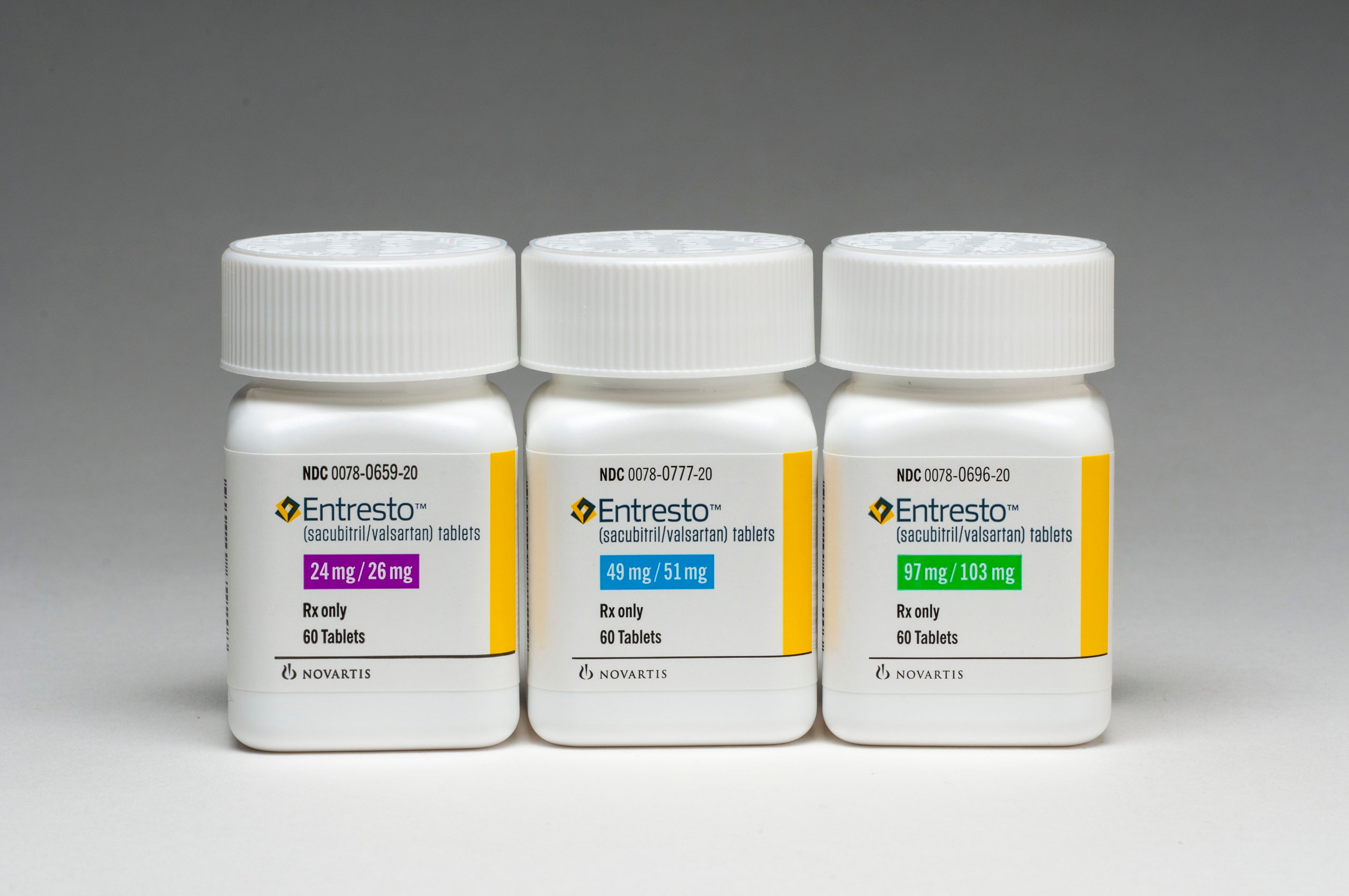
ENTRESTO
Marketed by: Novartis Pharmaceuticals Corporation
Indication: This combination of sacubitril and valsartan reduces the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction. It is usually administered in conjunction with other heart failure therapies, in place of an angiotensin-converting enzyme (ACE) inhibitor or other angiotensin II receptor blocker (ARB). The drug is not recommended for use in patients with histories of angioedema related to previous ACE inhibitor or ARB therapy or in those using aliskiren to treat diabetes.
Dosage Form:Tablets: 24 mg/26 mg, 49 mg/51 mg, and 97 mg/103 mg
For More Information:www.pharma.us.novartis.com

SAXENDA
Marketed by: Novo Nordisk
Indication: The FDA has approved Saxenda (liraglutide [rDNA origin] injection), a glucagon-like peptide-1 receptor agonist indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients. The prescribing information includes a black box warning for risk of thyroid c-cell tumors. The recommended dose of Saxenda is 3 mg daily, administered at any time of day without regard to meals.
Dosage Form:Solution for subcutaneous injection: prefilled, multidose pen that delivers doses of 0.6, 1.2, 1.8, 2.4, or 3 mg (6 mg/mL, 3 mL)
For More Information:www.saxenda.com
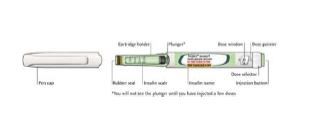
TOUJEO
Marketed by: Sanofi -aventis
Indication: The FDA has approved Toujeo (insulin glargine [rDNA origin] injection, 300U/mL) to improve glycemic control in patients with either type 1 or type 2 diabetes. Toujeo is 3 times more concentrated than traditional insulin glargine. As with any insulin, hypoglycemia is a potential adverse event.
Dosage form:Toujeo is only available in prefi lled SoloStar pens that deliver 1 to 80 units of insulin per subcutaneous injection. Each 1.5-mL pen contains a total of 450 units of insulin. Dosing is individualized based on clinical response.
For More Information:www.toujeo.com

