How to Safely Prescribe Upper Respiratory Symptom Relief for Hypertensive Patients
The provider treating a patient with both upper respiratory infection symptoms and hypertension must consider the side effects and potential drug interactions of the variety of symptomatic treatments available.
The provider treating a patient with both upper respiratory infection (URI) symptoms and hypertension must consider the side effects and potential drug interactions of the variety of symptomatic treatments available.1This article aims to assist the provider in making safe and therapeutically appropriate recommendations on symptomatic therapies for patients with URI. There are additional therapies available for patients with allergies that are not discussed in this article.
No attempt has been made to address antimicrobial stewardship, other than to recognize that antimicrobial prescribing has been identified to be suboptimal in many studies.2,3,4The prescriber or collaborating pharmacist is encouraged to conduct appropriate point-of-care testing for URIs and limit the use of non-indicated antimicrobials.5,6,7
Medications for symptomatic relief can be classified into 5 general categories: cough suppressants, antihistamines, decongestants, mucolytics, and pain relievers and antipyretics. Knowing when to recommend or prescribe these drugs requires an understanding of pharmacology, duration of drug action, patient discomfort, and potential drug—drug or drug–disease interactions.
The vast majority of URI symptoms can be, and are, treated with OTC products, often with no consultation with any member of the health care team. It is important that providers ask patients specific screening questions about their use of OTC products before prescribing or recommending any drug.8,9
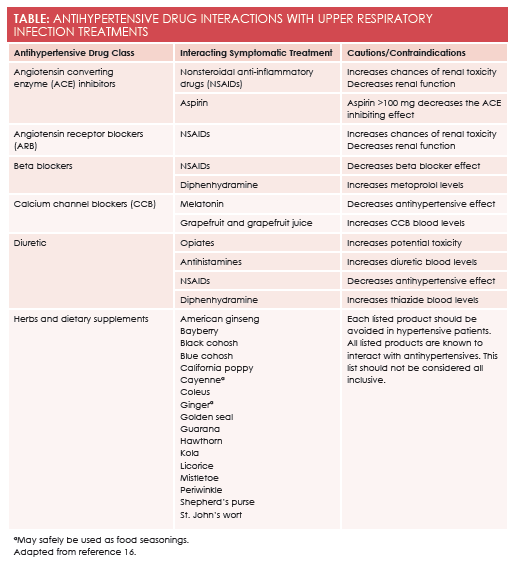
Cough suppressantsinclude dextromethorphan, opiates, and benzonatate. In this category, only dextromethorphan is routinely available without a prescription, although codeine-containing cough syrups may be available without a prescription in some states. There are limited cardiovascular contraindications to the use of available cough suppressants. Prescribers are reminded that opiate cough syrups have the potential for drug abuse and diversion. Opiates have been noted to increase the toxicity of diuretics. (Please seeTablefor drug—drug interactions). Dextromethorphan has also been used in an illicit fashion, particularly by teens and younger adults. Deaths from opiate overdose and dextromethorphan overdose have been reported. Benzonatate is not implicated in illicit drug behaviors. It is known to be sedating and is often recommended for bedtime cough suppression. Cough caused by angiotensin-converting enzyme (ACE) inhibitors is most appropriately treated by changing drug classes.10An ACE-induced cough will not respond to cough suppressants.
Antihistaminesare traditionally categorized as low sedation or higher sedation. All antihistamines used for URI symptoms are available over the counter. As a general rule, the higher the risk of sedation, the more likely the antihistamine is to cause drying. Dry nasal passages can lead to congestion, and dry mouth can lead to poor nutrition. Low-sedation antihistamines are effective against URI symptoms with less drying than diphenhydramine and other higher-sedation antihistamines. Antihistamine- induced xerostomia can be compounded by commonly prescribed antihypertensives including diuretics, beta-blockers, alpha-blockers, and calcium channel blockers. Diphenhydramine, in particular, may elevate levels of metoprolol and thiazide diuretics.
Sinus congestionis the symptom that brings hypertensive patients into community pharmacies and convenient care clinics more often than any other URI symptom, in part because of pain, and in part because of the well-publicized potential for decongestants to increase heart rate and blood pressure. Decongestants work by constricting the blood vessels of the sinuses; topical decongestants, such as oxymetazoline, cause rapid decongestion with limited systemic effects and can be used safely by hypertensive patients whose blood pressure is under control. Topical decongestants may only be used for 3 days and then must be stopped for at least 3 days. Cycling is allowed, but only for 3 cycles. Congestion lasting longer than 15 days must be evaluated by a specialist. The oral decongestant, pseudoephedrine, is a reliable decongestant available in 6-hour, 12-hour, and 24-hour duration products. Pseudoephedrine may be used orally on a daily basis, if required. Well-controlled hypertensive patients can safely take pseudoephedrine orally, but it should be used for the least amount of time possible.11,12
Mucolytic drugshave changed, dramatically, over the past 2 decades. At one time, iodinated compounds were the mainstay of mucous thinning. Today, the only routinely used mucolytic is guaifenesin. Interestingly, the benefit of guaifenesin is directly proportional to the patient’s hydration status, causing many authorities to claim that the best mucolytic is water. There are no identified cardiac or blood pressure concerns with guaifenesin, but there are identified benefits for the hypertensive patient when that patient is properly hydrated.
Pain and body achesare treated with analgesics and anti-inflammatory drugs. This decision is complicated for both the patient and the health care provider when the patient is being treated for hypertension or other cardiac conditions. All nonsteroidal anti-inflammatory drugs (NSAIDs) are accompanied by warnings for cardiovascular risk.13Acetaminophen does not carry these risks. Acetaminophen is the least likely to cause a drug—drug interaction and the least likely to cause stomach upset. Acetaminophen is the analgesic and antipyretic of choice for patients who are anticoagulated or using other thrombotic deterrents. When taken at labeled doses, it is safe. A careful evaluation of the patient’s cardiac risk and source of discomfort is essential to making a pharmacotherapeutically appropriate recommendation. If anti-inflammatory action is required, an NSAID or aspirin is best. The dose of aspirin must be carefully evaluated, as higher-dose aspirin can cause vasoconstriction, with doses as low as 100 mg decreasing the effectiveness of ACE inhibitors. Aspirin should be avoided in patients under the age of 21 years, when varicella zoster or influenza are possible causes of URI symptoms, due to their association with Reye’s syndrome. All OTC NSAIDs, aspirin, and acetaminophen are equally appropriate for managing fever. Only acetaminophen has no known drug– drug or drug–disease interactions when used in hypertensive patients. NSAIDs can decrease renal function and decrease the effectiveness of many antihypertensive therapies.
There have been few studies conducted on dietary supplements used to treat URI symptoms. There is very limited evidence that oral zinc may reduce the duration of the common cold in healthy adults; however, there are no data available to establish this as an appropriate recommendation for patients, particularly those with hypertension.14Many other dietary supplements have been studied with no identifiable benefit. A list of herbs and supplements known to interact with antihypertensives or the treatment of hypertension may be found at the end of the Table. Recent information highlights that dietary supplements can cause significant, even emergent, side effects, thus, these products should not be viewed as a safe alternative recommendation for the hypertensive patient.15
Allison M. Dering- Anderson, PharmD, RPh, is clinical assistant professor of pharmacy at the University of Nebraska College of Pharmacy in Omaha, Nebraska.Meagan Doyle is a PharmD candidate 2016 at the University of Nebraska College of Pharmacy in Omaha, Nebraska.
References
- Daniel H, Erickson S. Retail health clinics: executive summary of a policy position paper from the American College of Physicians.Ann Intern Med.Published online October 13, 2015. doi:10.7326/M15-0571.
- Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS).Infect Control Hosp Epidemiol. 2012;33(4):32-327. doi: 10.1086/665010.
- Reynolds B. How poor antibiotic prescribing puts patients at risk for deadly infections. Centers for Disease Control and Prevention website. http://blogs.cdc.gov/cdcworksforyou24-7/2014/03/how-poor-antibiotic-prescribing-puts-patients-at-risk-for-deadly-infections/. Published March 20, 2014. Accessed October 20, 2015.
- Havers F, Thaker S, Clippard JR, et al. Use of influenza antiviral agents by ambulatory care clinicians during the 2012—2013 influenza season. http://cid.oxfordjournals.org/content/early/2014/07/09/cid.ciu422.abstract.Clin Infect Dis. Published online July 16, 2014. doi: 10.1093/cid/ciu422.
- Gubbins PO, Klepser ME, Dering-Anderson AM, et al. Point-of-care testing for infectious diseases: Opportunities, barriers, and considerations in community pharmacy.J Am Pharm Assoc (2003). 2014;54(2):163-171. doi: 10.1331/JAPhA.2014.13167.
- Burley E, Klepser S, Klepser M. Opportunities for pharmacists to improve access to primary care through use of CLIA-waved tests.Michigan Pharmacist. 2014;52(2):8-11.
- Rodis JL, Thomas RA. Stepwise approach to developing point-of-care testing services in the community/ambulatory pharmacy setting.J Am Pharm Assoc (2003). 2006;46(5):594-604.
- Klepser ME. Socioeconomic impact of seasonal (epidemic) influenza and the role of over-the-counter medicines.Drugs. 2014;74(13):1467-1479. doi: 10.1007/s40265-014-0245-1.
- Klepser DG, Corn CE, Schmidt M, Dering-Anderson AM, Klepser ME. Health care resource utilization and costs for influenza-like illness among midwestern health plan members.J Manag Care Spec Pharm. 2015;21(7):568-573.
- Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines.Chest.2006;129(1 suppl):169S-173S. doi:10.1378/chest.129.1_suppl.169S.
- Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate: a meta-analysis.Arch Intern Med. 2005;165(15):1686-1694.
- Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension?J Fam Pract. 1995;40(1):22-26.
- NSAIDs and cardiovascular risk explained. ScienceDaily. www.sciencedaily.com/releases/2012/05/120502143846.htm. Published May 2, 2012.
- Singh M, Das RR. Zinc for the common cold.Cochrane Database Syst Rev. 2013;6. doi: 10.1002/14651858.CD001364.pub4.
- Geller AI, Shehab N, Weidle NJ, et al. Emergency department visits for adverse events related to dietary supplements;N Engl J Med. 2015;373:1531-1540. doi: 10.1056/NEJMsa1504267.
- Lexi-Interact [online database]. Hudson, OH: Lexicomp Inc; 2015. http://online.lexi.com. Accessed October 2015.

